Michelle Brady remembers how much her daughter Addie loved life.
“She was an incredible young child. Very bright, very, very beautiful. I know a lot of parents say that about their children, but she truly was.”
Addie had a rare form of bone cancer. Her tibia had to be replaced with a prosthetic bone in her leg and she spent months in hospital enduring harsh chemotherapy. But it wouldn’t be her last brush with cancer.
“When she was 14, she got up one morning and just out of the blue — there was no signs of any of this, this is five years on from her first cancer — she was unable to speak and she had a seizure,” Michelle says.
“And she was rushed to hospital by an ambulance. A couple of days after that, we were advised that she had an inoperable brain tumour.”
Michelle knew something more was going on. It didn’t make sense that her young daughter could have two different cancers in such a short time.
Genetic testing revealed Addie had Li Fraumeni syndrome — or LFS.
It’s an inherited mutation to the TP53 gene, which is known as the “guardian of the genome” because it stops cancers from forming. But for people with LFS, this process goes awry.
“Addie knew the seriousness of cancer because at nine years old, she had experienced it,” Michelle says.
“She’d seen children that she’d been on the ward with lose their lives to cancer.
Key points:
- Addie Brady was diagnosed with cancer at age 9 and age 14
- Genetic testing revealed she had an inherited mutation to the TP53 gene, known as the “guardian of the genome”
- Researchers have high hopes for what precision medicine can do for those born with cancer risks
Even as Addie had cancer treatment at the age of nine, Michelle says she was selfless and brave.
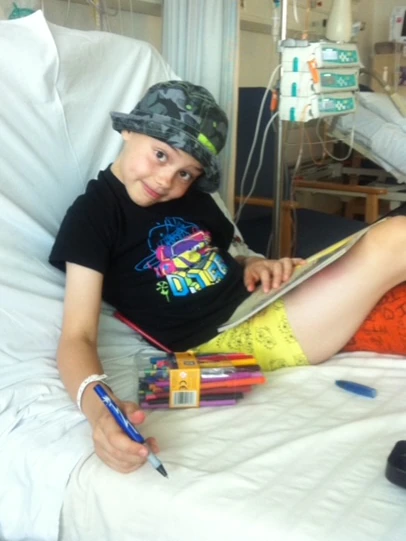
Addie had an inherited mutation to the TP53 gene, which is known as the “guardian of the genome”.(Supplied: Michelle Brady)
“During the time before we did lose her, she asked me probably twice, ‘Am I going to die, Mum?’ And it was one of the toughest questions I’ve ever had to answer.”
The cancer eventually moved to Addie’s spine and Michelle remembers how hard the last few months of Addie’s life were.
“It was heartbreaking to watch her experience that … and not being able to do anything about it.”
Addie died in February 2018, aged just 16.
While LFS is usually inherited, Addie had what’s known as a de novo mutation — meaning she was the first person in the family to carry the mutation.
In the past few decades, many hereditary mutations have been discovered that put people at risk of cancer.
There are genetic changes that occur in all cancers, often because of lifestyle and environmental factors. But these gene changes are inherited – not acquired.
An inherited gene mutation does not guarantee cancer will develop, but it does put people at much higher risk, often at a younger age. These inherited mutations are behind 5-10 per cent of all cancers.
Doctors searching for ‘targeted treatments’
The Children’s Cancer Institute’s Dr Mark Pinese leads a personalised medicine team that is looking at the inherited and do novo gene variants that put some children at risk of cancer.
“If we can identify those kids who are at risk of cancer, then in the future we might be able to have … relatively minor drugs that they can take, that will take away their risk … that they just don’t develop cancer at all,” Dr Pinese says.
“And that for us is the ultimate goal — and so much better than a cure.
Researchers like Dr Pinese have high hopes for what precision medicine can do for those born with cancer risks.
Currently, childhood cancer treatment is often harsh and unspecific, because much of the research to date has been done on adults.
“Very sadly, many children who survive cancer do so with long-term side effects,” Dr Pinese says.
“These can be lifelong effects on their fertility, on their growth and development, on their learning.
“If we can find targeted treatments, we believe that that will minimise side effects as well.”

‘We call it scan-xiety’
Another tool being used is surveillance, with a clinical trial currently being run across multiple states in Australia.
Known as SMOC, it’s using MRIs and other surveillance for people at high risk of developing cancer — particularly multiple types of cancer, like those with Li Fraumeni syndrome.
“If we take a very active approach, we can actually make a difference, even in a condition that has such a high risk like LFS,” says clinical geneticist Professor Paul James, the director of the Familial Cancer Centre at the Peter MacCallum Cancer Centre and Royal Melbourne Hospital.
Anna has already had cancer three times – but none were related to the mutation.
“[My son] was only six years old when I was first diagnosed with cancer. [He] saw his mother sick,” she says.
“My biggest fear was that my son was going to have it (the gene mutation). And unfortunately, he does.
“But like I said to him, we have to think of it as a blessing that we know about it and we’re both being tested.
“So if something does turn up or reappears some way, it will be caught early.”
“We call it ‘scan-xiety’. I mean it’s always going to be there, that anxiety in the back of your head like, ‘oh, what if it’s come back?’
“It’s like you look in the mirror and you see scars from surgery. I don’t go a day without thinking of it.”
There used to be a view in medicine that knowing these genetic risks was often futile because not enough was known about how to prevent or treat associated cancers.
But Professor James says that’s shifting.
“That fatalistic view has really been replaced by a view that if we know who is at risk, then there’s much more that we can do.”
But despite how far precision medicine has come, it’s still only in its infancy. Much of what is known about hereditary risk focuses on single-gene mutations.
Professor James says researchers are also turning their attention to how multiple genes interact with each other.
“So instead of the risk being due to one large genetic fault that occurs in a single gene, we’re starting to think about the way that genetic variations across the genome start to interact with each other.
“It’s known as polygenic risk, and that’s just starting to come through now as something that we’re beginning to be able to look at, at least in the research setting.”
Meanwhile, Michelle Brady continues her daughter Addie’s legacy by supporting families and research into hereditary cancer syndromes like LFS.
“A lot of people say to me, ‘how do you cope? How do you do this?’
“And I do it because I know that she wouldn’t want me to carry on through life just mourning her death.
“She’d want me to do something because of what happened to her.”

ABC Radio Interview
You Cannot Be Serious Podcast with Sam Newman and Dr Nicole Yap
You Cannot Be Serious - Episode 134 - Doctor Doctor Give...
RPPV News 05.02.2022
https://youtu.be/wjdoQdWSyQI



