Among women, breast cancer accounts for over 25% of cancer diagnoses and 15% of cancer- related deaths. The majority of cancers are sporadic. They are caused by the progressive accumulation of genetic mutations and/or epigenetic changes during a person’s lifetime. These are not heritable, that is passed down from one generation to another.
5-10% of women with breast cancer have a family history of the disease. Compared with women without a family history, women with one premenopausal first-degree relative with breast cancer are at 3.3 fold greater risk, and women with two first-degree relatives with breast cancer are at 3.6 fold greater risk of developing a breast cancer in their lifetime
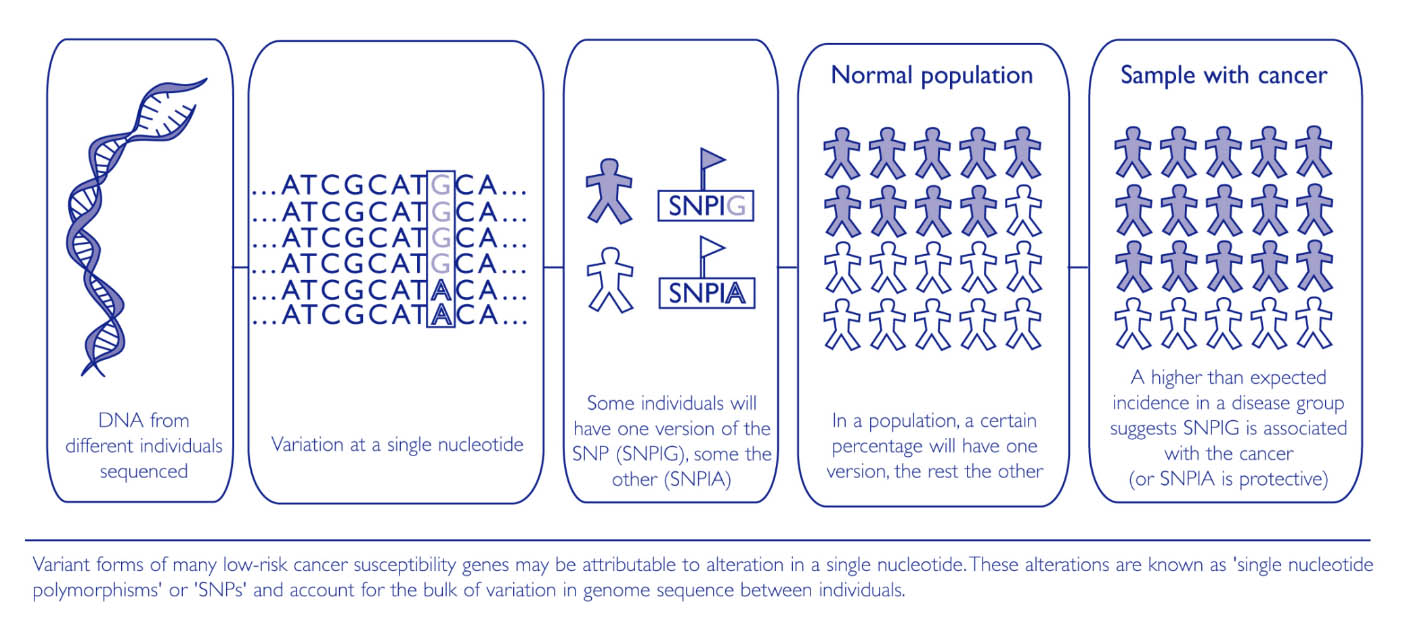
Certain inherited, natural variations in our genes (known as polymorphisms) may also influence the risk of developing a sporadic cancer.
A number of polymorphisms that affect the risk of developing different types of cancer have already been identified.
This search is being facilitated by the availability of the human genome sequence and the development of high-throughput single nucleotide polymorphism (SNP) array technology.
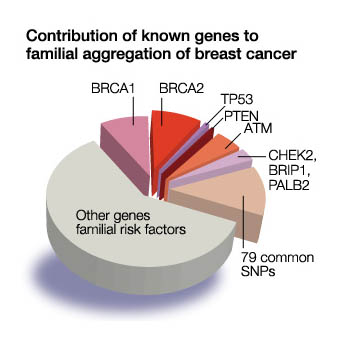
The most common cause of hereditary breast cancer is an inherited mutation in the BRCA1 or BRCA2 genes. These are breast cancer suppressor genes, which repair damaged DNA, located on chromosome 17 and 13 respectively. When these genes are mutated or damaged, cell growth can be abnormal and thus lead to cancer. BRCA1 andBRCA2 mutations account for 5 to 10 percent of breast cancers and 10 to 15 percent of ovarian cancers
Mutations in the BRCA1 and BRCA2 are inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to increase a person’s chance of developing cancer. That means every offspring of one affected parent, has a 50% chance of receiving the mutated gene.
Women with BRCA1 orBRCA2 are about five times more likely to develop breast cancer than a woman who does not. The cancer tends to be more biologically aggressive, high grade and occur in younger age groups (ie < 50 yrs).
Lifetime risk estimates for ovarian cancer among women in the general population indicate that 1.4 percent (14 out of 1,000) will be diagnosed with ovarian cancer compared with 15 to 40 percent of women (150–400 out of 1,000) who have BRCA1 or BRCA2 mutation
BRCA1 mutations also increase the risk of cervical, uterine, pancreatic, and colon cancer.
BRCA2 mutations increase the risk of pancreatic cancer, stomach cancer, gallbladder and bile duct cancer, and melanoma .
The BRCA2 gene mutation has an increased risk of male breast cancer, as well as pancreatic cancer and prostate cancer in men.
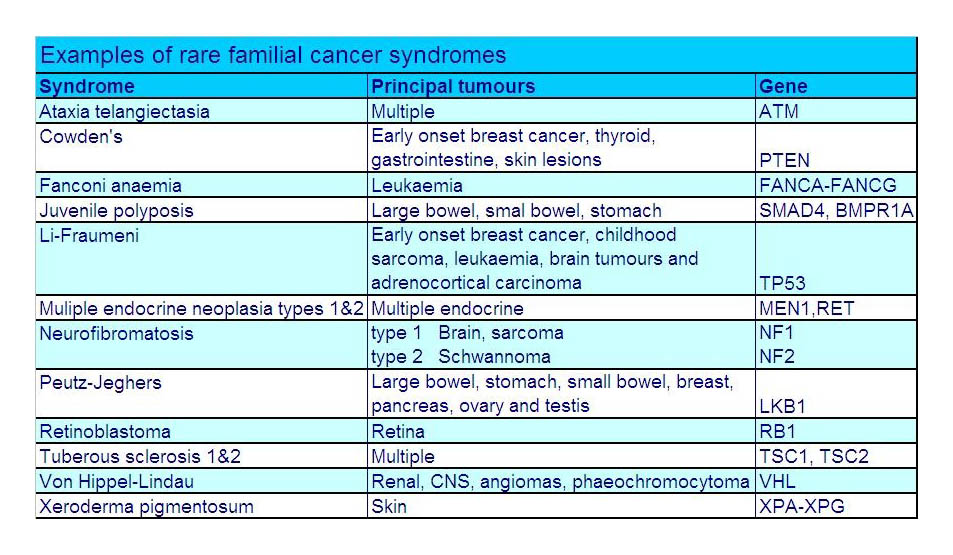
Mutations in several other genes, including TP53, PTEN, STK11/LKB1, CDH1, CHEK2, ATM, MLH1, and MSH2, have been associated with hereditary breast cancers. These can be associated with particular subtypes of breast cancer and syndromes. CDH1 is associated with invasive lobular carcinoma (tends to be bilateral breasts and multifocal), and the highly aggressive subtype , triple negative (not responding to Eostrogen, Progesterone, or Her-2) associated with mutations on RAD51C, RAD51D, BARD1.
Syndromes associated with breast cancer are Li-Fraumeni syndrome (mutation in TP53); Peutz-Jeager Syndrome (STK11); Fanconi anaemia (PALB2); and Ataxia -telangiectasia (ATM).
Management of High Risk Patients
Strong family history of breast/ovarian cancer and/or carrier of genetic mutation or increased single-nucleotide polymorphism (SNPS)
If there is a strong family history of first and/or second degree relative with breast and or ovarian cancer under the age of 50 yrs, male with breast cancer in the family, Ashkenazi Jewish descent, then consideration must be made regarding management of the family members with no breast symptoms.
The three approaches can described as regular surveillance, chemoprevention, and prophylactic risk reduction surgery. Genetic testing is also part of the management plan. If the genetic mutation test is negative, it DOES NOT imply that there is no genetic mutation, but rather there MAY be no genetic mutation as we have not tested for it!
Increased Surveillance
Rigorous monitoring aims to diagnose very early breast cancers, and as hereditary breast cancers tend to be aggressive, the time frame should be 6 monthly, and commenced at least 10 years prior to the youngest diagnosed member of the family. In other words, if the youngest was diagnosed at 40 years of age, then other family members should commence monitoring at 30yrs of age. Monitoring requires history, clinical examination, and in my hands, alternating mammogram/breast ultrasound, with MRI breast. From 50 years of age, if the breast tissue is less dense, I replace MRI with Ultrasound imaging due to losing MRI rebate from Medicare.
If there is also a family history of ovarian cancer, I also suggest an annual blood test for ovarian tumour markers Ca 125, and Transvaginal Ultrasound.
Chemoprevention is the use of Tamoxifen (an anti oestrogen), to reduce the occurrence of Breast cancer but is ineffectual again triple negative disease, of which is the biology of many breast cancers associated with genetic mutations. It is therefore thought to be <50% effective. Consideration must also be made regarding the risk reduction versus the potential side effects of deep vein thrombosis and increased risk of endometrial (uterine) cancer.
Definitive management is risk reduction surgery. This allows for 98% reduction of being diagnosed with a cancer as the effective tissue is removed.
Regarding breast tissue, a bilateral mastectomy is performed, either a traditional approach, leaving a flat chest without a nipple-areolar complex, or skin sparing/nipple-areolar sparing with reconstruction.
In BRCA 1 and 2 mutations, consideration must also be taken of ovarian pathology, as early ovarian cancer is difficult to diagnose, and late stage ovarian cancer carries a poor prognosis. Consideration is given to fertility issues with tumour markers and ultrasound annually, until prophylactic surgery which is a bilateral salpingo-oophorectomy (removal of the ovaries and associated tube) is considered. This can be hastened if egg retrieval and storage is considered. This allows for fertility in the long term, whilst removing the potential risk of cancer in the ovarian tissue.